ADME / TOX Screening
Discovery PK & Non-GLP Services

Strengthening regulatory readiness with timely early-stage studies
Screen PK
Frontage offers fast and reliable PK screening services using mouse and rat. Screening PK provides the initial data of in vivo exposure and clearance of a compound, an essential step before the subsequent animal efficacy and PD studies.
Adopting efficient PK studies during discovery helps in choosing the right lead candidate. PK screening is used in preclinical efficacy species to determine if a compound has the necessary exposure to achieve efficacy following in vivo dosing. Such screening studies may help in elucidating if the compound in question is specific to the target and if it has enough systemic exposure for efficacy. When studies are performed in combination with in vitro ADME studies, candidates with poor absorption, inability to reach the therapeutic target, potential for cross-interactions can be screened out. For later-stage drug candidates, Frontage offers dog and monkey PK services.
Mini AMES
Frontage offers non-GLP mini Ames tests in which Salmonella cell lines are used to monitor the mutagenic potential of the test compounds. Most compounds that are found to induce genetic mutations in preclinical models are often discontinued for further efforts due to the dramatically increased risk of toxicity. The only rare exceptions are certain life-saving oncology drug candidates.
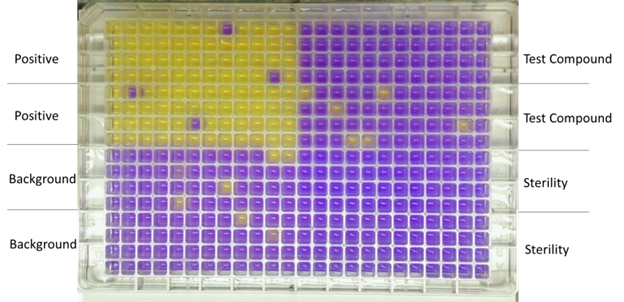
Salmonella strains cannot grow in histidine-free medium unless mutations occur in certain genes. The mini Ames assay is performed in 384-well plates using two Salmonella strains: TA98 (frameshift mutation) and TA100 (base-pair substitutions). The Ames test can be carried out in the presence and absence of a metabolizing system to identify potential mutagenicity by the parent compound and its metabolites.
hERG
The binding of compounds to the cardiac potassium channel encoded by the human ether-a-go-go-related gene (hERG), will result in reduced function of hERG, lengthens ventricular action potentials, prolongs the QT interval in an electrocardiogram, and increases the risk for potentially fatal ventricular arrhythmias, and sudden death. At Frontage, we developed manual patch clamp assay for measuring hERG activity.
Non-GLP Bioanalytical Services
Frontage offers quick turnaround of LC/MS/MS based bioanalytical services in plasma/serum/whole blood and tissue analysis of small molecules as well as ADCs, peptides and proteins.
Formulation Testing
In pre-clinical pharmacokinetic (PK) and toxicokinetic (TK) studies, formulations play a critical role to ensure test articles are completely dissolved for intravenous administration and properly absorbed for oral or other routes of administration. Frontage has helped many companies in formulation development using different strategies based on the properties of the new API. Our primary approaches are:
- The evaluation of pH effect
- Selection of co-solvents
- Selection of surfactants
- Complex formation using cyclodextrin or its derivatives
- Lipid-based formulations
- Combinations of multiple strategies above
Resources To Consider


Global Drug Discovery Services Overview

Frontage Hayward, CA Site Virtual Tour

Chemistry Services


