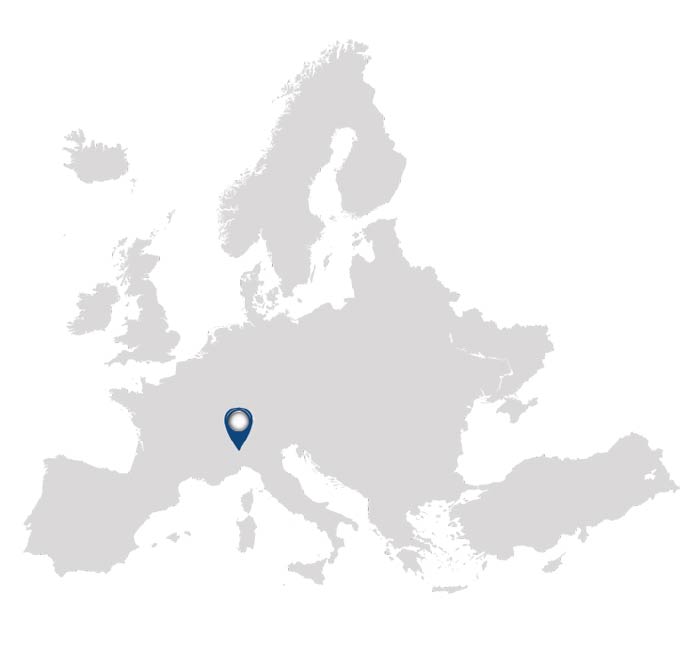
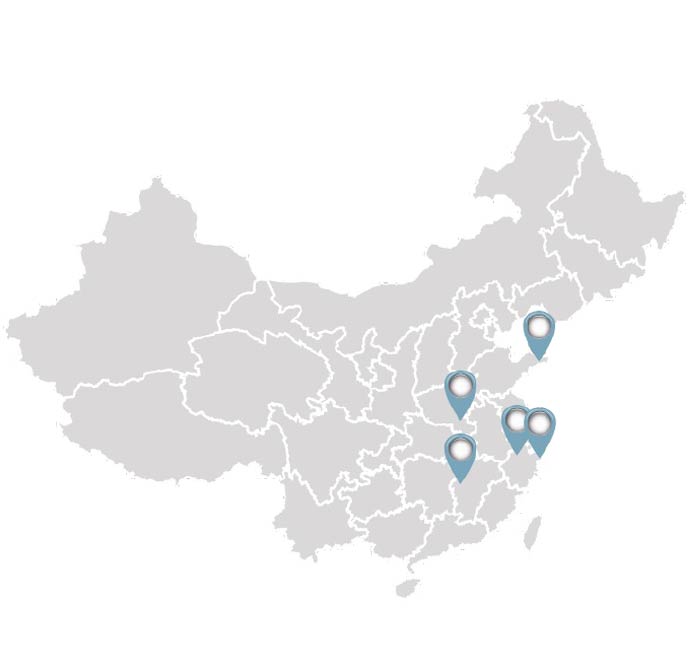
Evolution of a CRO
Frontage Laboratories, Inc. is a global pharmaceutical development organization advancing drug discovery and development through integrated laboratory and clinical services. More than just a CRO, we assume role of drug developer, assisting clients in advancing hundreds of molecules through development to commercial launch in global markets. Our commitment to providing rigorous scientific expertise to ensure the highest quality and compliance to allows us to deliver efficient and cost-effective development of new drugs.
We have enabled many innovators, generic, and consumer health companies of all sizes to file IND, NDA, ANDA, BLA, and 505(b)(2) submissions in global markets allowing for the successful development of important therapies and products for patients.
Successful partnerships with hundreds of drug development companies across the globe to bring innovative therapies to patients.
- Over 20 years of successful growth.
- Double-digit revenue growth over last 10 years.
- Industry recognized for strong science,quality, regulatory,
and state-of-the-art technology. - Clients range from start-ups to Top-20 pharma companies.
The Value We Bring To Our Clients
We are a value-added partner focusing on solving our customers’ most significant and complex drug discovery and development challenges. Frontage’s scientific knowledge base, technical expertise, and reputation for high-quality services have been integral to our ability to enter into long-term solid strategic relationships and partnerships with our key customers.

SCIENTIFIC
EXPERTISE
Consultation and collaboration with our clients to solve complex drug development challenges by leveraging our strong scientific expertise.
HARMONIZED GLOBAL QUALITY STANDARDS
Application of consistent high-quality standards, systems, and process across all sites globally ensures data integrity for regulatory review,decision support, and adjudication.
LEVERAGING STATE OF
THE ART TECHNOLOGY
Apply our deep understanding of laboratory science with our best-in-class platforms to develop solutions to complex scientific challenges.
Comprehensive Drug Discovery & Development Services
Frontage Laboratories offers a comprehensive suite of drug discovery and development services including chemistry, DMPK, preclinical safety/toxicology, and clinical trial services with strategic and regulatory support to effectively bring new lead candidates through the IND stage to Phase II.
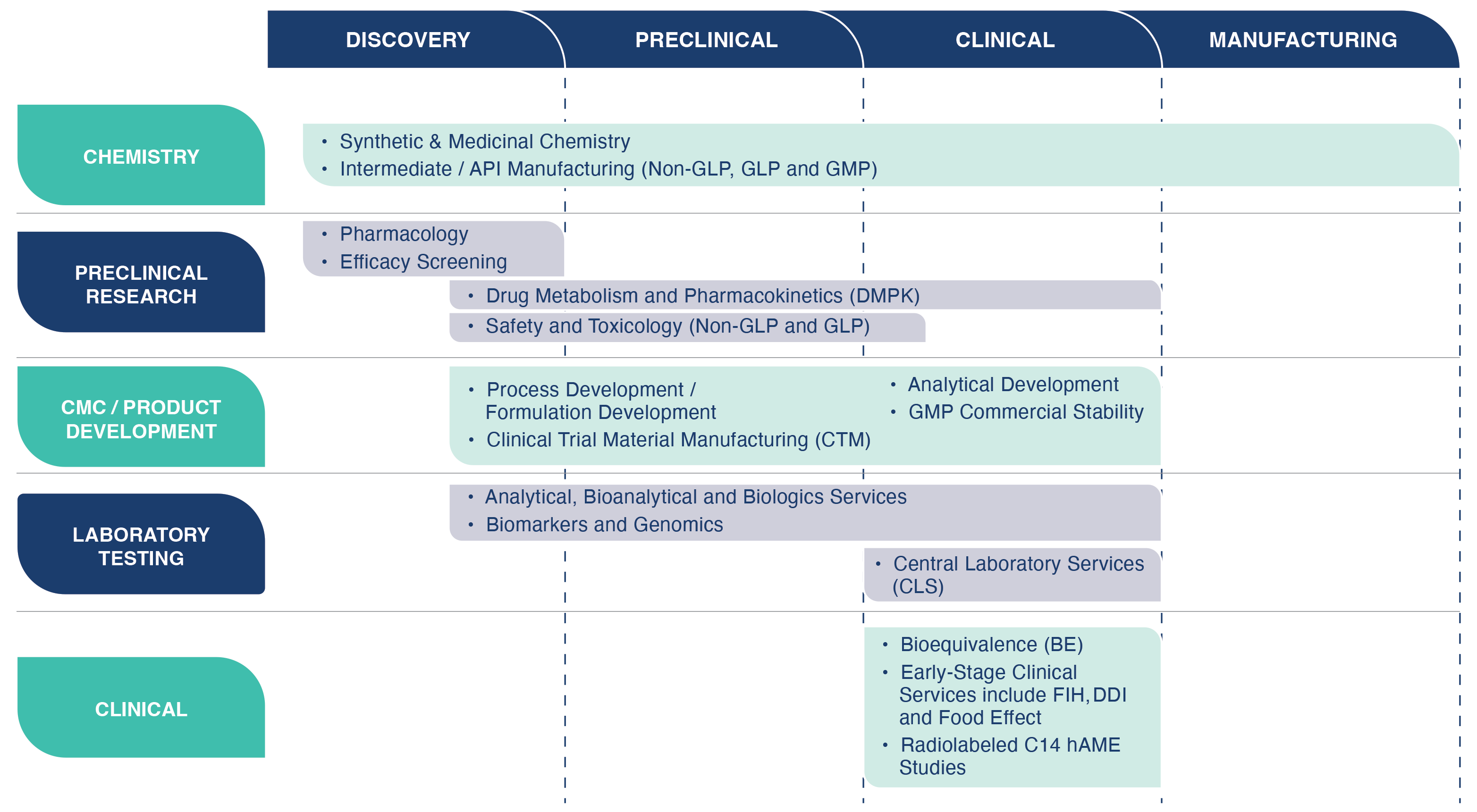
Creating a New Paradigm with Integrated Service Programs
Our Integrated Service Programs takes your drug candidates from lead optimization through first in human (FIH) studies. We offer integrated service programs to compress timelines, mitigate risk, and reduce costs. As your drug development partner, we offer scientific and regulatory strategic consulting. We conduct all aspecdts of the required testing leading to preparation and submission of an IND application, FDA interacactions, and Phase 1 clinical studies.
DISCOVERY
Medicinal Chemistry, Pharmacology/Biology, and ADME/Tox Screening in NA and China.
DRUG DEVELOPMENT
Strong science driven DMPK, Safety & Toxicology and Clinical organizations that can provide end-to-end development services.
LAB TESTING SERVICES
Regulated (GLP/GMP) Bioanalytical and Analytical Chemistry services to support Safety Tox, and Clinical Studies.
PRODUCT DEVELOPMENT & MANUFACTURING
API Synthesis Formulation and Clinical Trial Material Manufacturing (CTM).