Biologics Cell & Gene Therapy
Frontage’s Biologics, Cell and Gene Therapy (BCGT) division is staffed by leaders and scientists with specialized skills in biopharmaceutical development, bringing together a wealth of experience from major biopharmaceuticals, biotech, CDMOs, and CROs.
Learn more about Cell and Gene Therapy
A ‘one-stop-shop’ experience.
Our service in product characterization, analytical method development, and method qualification/validation are performed within a single facility. Our state-of-the-art laboratory, adhering to GLP/GMP standards, has advanced instruments for efficient method development and execution.
Frontage has developed a robust approach to drug development, accommodating method transfer and development for drug substances and products to ensure products’ safety, potency, identity, strength, and purity.
We also provide platform methods to support early-stage projects and to be tailored for your specific drug development. We look forward to exploring how our expertise can enhance and expedite your drug development program.
Biologics Services
CMC biologics is experienced in supporting projects from candidate selection to commercial product.

Cell & Gene Therapy Services
Cell therapy represents a groundbreaking frontier in modern medicine.

GMP Routine Testing Services
Supporting clients’ biologics and cell and gene therapy (CGT) pipelines through various stages of drug development.

Stability Program
ICH Compliant Stability Facility for Cell and Gene Therapy Products.
Method development, qualification, and validation are at the core of our service portfolio.
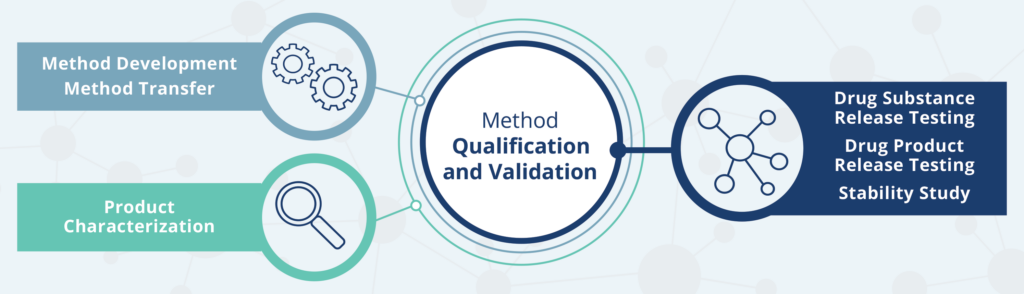
From product characterization to first-in-human to later stages of your drug development/approval cycle, we can support the following:
- Assay development and technology transfer.
- Method qualification and validation for GMP release
- Long-term stability studies for your drug substance and drug products

The Frontage Advantage
The Biologics, Cell and Gene Therapy team at Frontage Laboratories comprises seasoned leaders that support product development, with experience spanning from IND-enabling studies to BLA approval. This team is composed of highly skilled scientists that prioritizes quality, collaboration, and service.
Our experts are dedicated to working with our clients to develop and refine analytical testing methods and procedures to advance the development of the client’s products.
Resources To Consider

Gene & Cell Therapy Analytical Services
Biologics Analytical Testing Services


