In Vivo Services
Human C14 AME (hAME) Study Services
 Services & Solutions
/
Drug Metabolism & Pharmacokinetics (DMPK)
/
In Vivo Services
/
Human C14 AME (hAME) Study Services
Services & Solutions
/
Drug Metabolism & Pharmacokinetics (DMPK)
/
In Vivo Services
/
Human C14 AME (hAME) Study Services

Full-Service Radiolabeled hAME Studies
Human absorption, metabolism and excretion (hAME) study is conducted to identify compound disposition in the body and to characterize its metabolites.
When it comes to radiolabeled hAME studies, Frontage is your one-stop-shop. The combination of our in-house clinical and DMPK experts provides our clients full support, with a comprehensive approach from initial planning for their hAME study through final study report.
Frontage Collaborates With Clients To Design Studies That:
- Determine the pharmacokinetics, routes of elimination and clearance of a potential therapeutic agent.
- Identify metabolites in various biological matrices using high-resolution mass spectrometry, other analytical techniques such as high-field NMR, and by comparison with synthetic standards.
- Obtain the relative exposure of major metabolites and confirm adequate coverage in preclinical toxicology species.
- Maximize cumulative recovery of total radioactivity in excreta (mass balance).
Frontage Labs Highly-Experienced DMPK Team Approach:
- Following Quantitative Whole-Body Autoradiography (QWBA), a radiation dosimetry report that is based on current regulatory recommendations is provided prior to hAME study conduct.
- A detailed analytical protocol is provided for the analysis of samples to support the hAME study.
- Daily reporting of mass balance data to demonstrate recovery and excretion pathways of administered radioactivity. Furthermore, daily mass balance data to ensure the timely release of subjects who meet the discharge criteria.
- DMPK-to-Clinical transition of information within Frontage fosters minimal delays in reporting data and enhances timeline efficiencies.
- Metabolite identification, characterization, and profiling of samples by the industry’s leading experts equipped with state-of-the-art instrumentation.
Sample Timeline for hAME Study
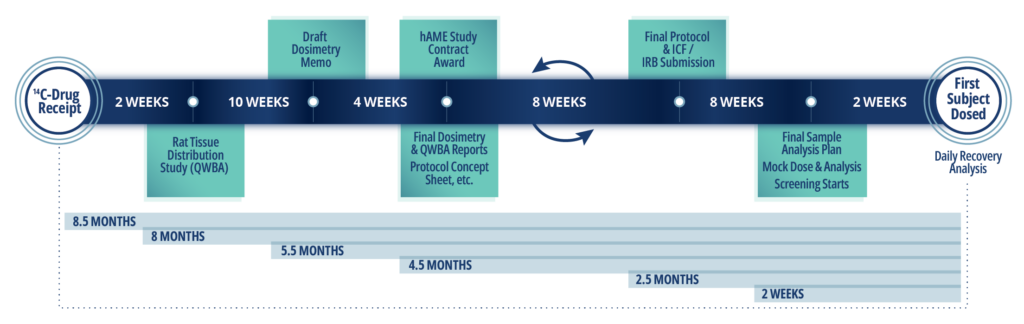
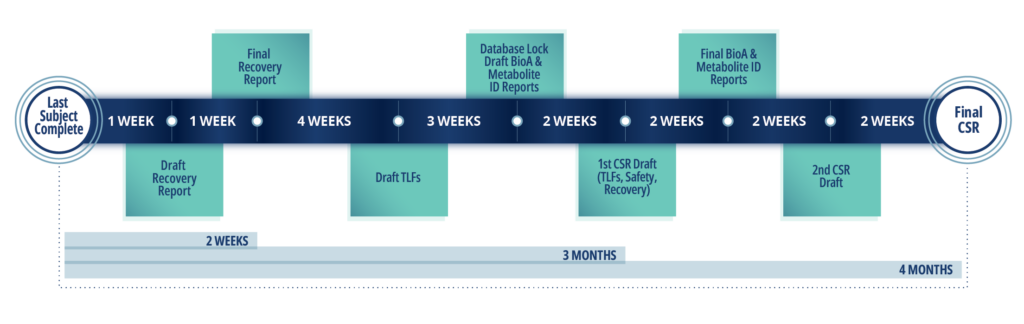
Discover the following In Vivo services at Frontage

Pharmacokinetics
Pharmacokinetics studies to investigate new chemical entities.

MIST – Metabolites in Safety Testing
A key component of human risk assessment for drug candidates.

Radiolabeled Studies

QWBA Studies
Evaluating the time course of elimination for total radioactivity from tissues in animals.

Human C14 AME (hAME) Study Services
Full-Service Radiolabeled hAME Studies
Resources To Consider


Full Service Radiolabeled Human C14 AME (hAME) Studies

Quantitative Whole-Body Autoradiography (QWBA) and Radiation…

MIST Application Note

