Clinical Study Experience
Human 14C AME Study Services
IN THIS SECTION
Frontage combines our in-house clinical and DMPK expertise to provide our clients with a comprehensive approach from initial planning for their human AME (hAME) study through the final Clinical Study Report.
A 14C AME study uses a radiolabeled compound to evaluate how the study drug is absorbed, metabolized, and excreted after dosing. The results of such studies elucidate the pharmacokinetics, mass balance, metabolic pathways, and more. hAME studies are typically required for New Drug Applications (NDA) and for some Biologics License Applications (BLA).
Frontage Clinical Services Approach
- Purpose-built clinical facility with a dedicated, restricted access unit to conduct hAME studies.
- Expert hAME study protocol development/review, along with data management and biometrics support services.
- Rapid recruitment from a large database of healthy volunteer subjects, or patient populations and studies conducted by a highly skilled and experienced research team.
- Experienced nuclear pharmacist to prepare doses on-site for oral or injection administration
- Collection and processing of subject blood and excreta.
- Seamless integration with Frontage DMPK for sample analysis and real-time radioactivity counting that allows for timely subject release.
Frontage Labs Highly Experienced DMPK Team Approach
- Following Quantitative Whole-Body Autoradiography (QWBA), a Radiation Dosimetry Report based on current regulatory recommendations is provided to support hAME study protocol preparation.
- A detailed analytical protocol is generated for the analysis of samples.
- Daily reporting of mass balance data to demonstrate recovery and excretion pathways of administered radioactivity, and to ensure the timely release of subjects who meet the discharge criteria.
- DMPK-to-Clinical transfer of information within Frontage allows for minimal delays in reporting data and enhances timeline efficiencies.
- Metabolite identification, characterization, and profiling of samples by the industry’s leading experts equipped with state-of-the-art instrumentation.
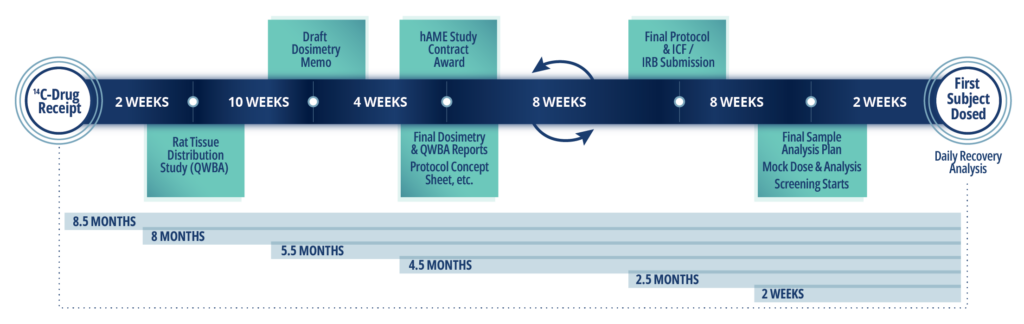
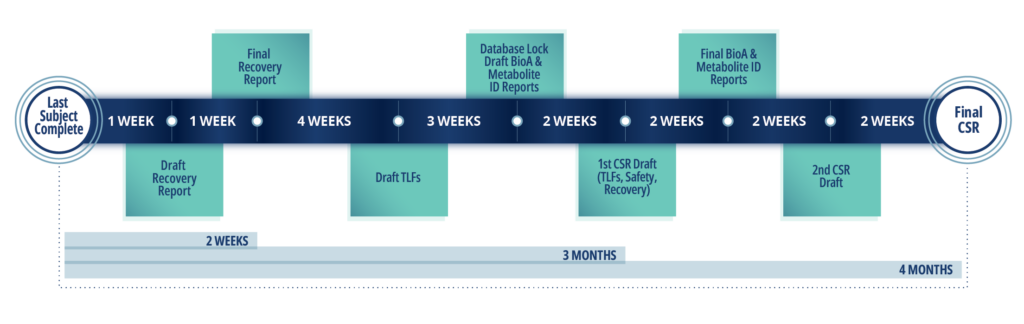
Sample Timeline for hAME Study
Frontage hAME Team
- Expert staff with an average of 25 years of experience in QWBA, dosimetry, hAME, and early phase clinical studies.
- A science-driven team to provide input to study design and interpretation of results to deliver high-quality data in a timely manner.
- Proven history of flexibility, focus on communication, and successful execution of all pre-clinical and clinical studies.
Resources To Consider


brochure
Full Service Radiolabeled Human C14 AME (hAME) Studies
Frontage’s Clinical and DMPK teams work together seamlessly to provide full-service radiol…

webinar
Quantitative Whole-Body Autoradiography (QWBA) and Radiation…
This webinar gives an overview of QWBA and hAME study services, an understanding of the gu…

fact-sheet
DMPK Services Fact Sheet
At Frontage, we offer extensive drug metabolism and pharmacokinetic capabilities for new c…


