Services
Immunogenicity

An immunogenicity assay serves as a crucial tool in evaluating the body’s immune response to various substances, such as vaccines, drugs, or biological therapies.
By measuring the presence and magnitude of immune reactions, this assay aids in assessing the effectiveness, safety, and potential risks associated with the tested compounds.
Immunogenicity assay includes anti-drug antibody (ADA) assay and neutralizing antibody (NAb) assay. ADA assay is designed to detect antibodies generated by the immune system in response to a specific drug or therapeutic protein. These antibodies, known as anti-drug antibodies, can impact the efficacy and safety of the drug by altering its pharmacokinetics and pharmacodynamics. The NAb assay is specifically designed to measure the presence of antibodies that neutralize the biological activity of a drug or therapeutic protein. Neutralizing antibodies can directly inhibit the function of the target molecule, thereby affecting treatment efficacy and safety.
Immunogenicity Assay Workflow
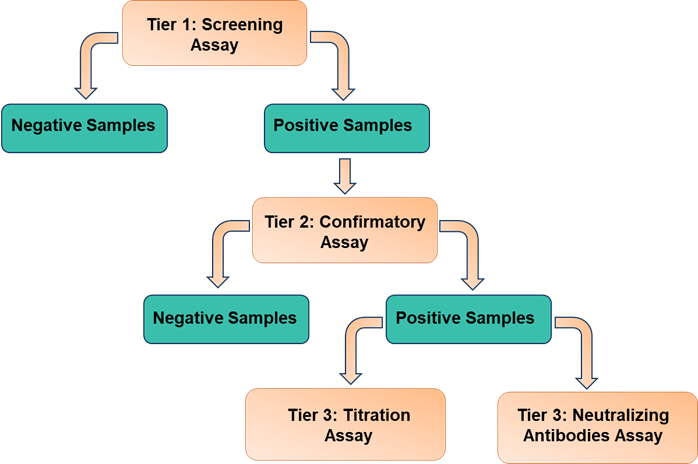
Immunogenicity Assay Format (Ligand Binding Assay)
- Bridging method
- Affinity capture elution (ACE) method
- Solid phase extraction with acid dissociation (SPEAD) method
- Customized method
Additional Services to Explore:

PK/TK Studies
Frontage’s bioanalytical team has earned a reputation for consistent and high-quality delivery

Immunogenicity
An immunogenicity assay serves as a crucial tool in evaluating the body’s immune response to various substances

Sample Management
An optimized sample management process

FTE Services
Frontage offers service FTE services models to support your bioanalysis program
Resources To Consider


Pre-Existing Antibodies within Immunogenicity Testing

Testing of Carryover & After MRD Benchtop Stability for…

Achieving sensitive and specific oligonucleotide quantification by…


