Genomic Services for Oncology
ctDNA Analysis
Frontage offers analysis of cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA) for the detection and quantification of somatic mutations in cancer-associated genes, allowing for disease detection and monitoring with minimally invasive strategies.
Isolated cfDNA can be examined using targeted sequencing with our NGS Oncology Panel, which allows for the discovery of almost unlimited variants in a large set of cancer-associated genes. Alternatively, ddPCR allows for a more focused approach to identify specific variants in a smaller subset of genes.
Extraction and Purification of cfDNA
Frontage offers an optimized and fully validated workflow for cfDNA extraction and purification. Our automated cfDNA extraction method allows for high-throughput sample processing and quick turnaround times. Manual cfDNA extraction is also available for difficult samples or smaller batches. An additional purification step is used for the removal of contaminating genomic DNA molecules to enrich for ctDNA. Our cfDNA workflow allows for the isolation of high-quality ctDNA for downstream variant detection and analysis.
Size distribution of cfDNA isolated from healthy plasma

Bioanalyzer traces of cfDNA isolated from healthy plasma samples (top) and the corresponding Illumina DNA sequencing libraries (bottom), showing the expected DNA fragment size distribution.
Variant Detection from ctDNA using ddPCR
The genomics team at Frontage has expertise in variant detection using ddPCR, including extensive experience in custom assay design and assay optimization. When detecting specific variants in a smaller subset of genes, ddPCR allows for a more focused approach with high sensitivity.
NGS Oncology Panel
Frontage offers a laboratory developed test (LDT) that uses NGS technology and targeted sequencing to simultaneously detect somatic variants in hundreds of cancer-associated genes from ctDNA. Our fully validated NGS oncology panel and cfDNA workflow is fully validated for clinical use under CLIA/CAP.
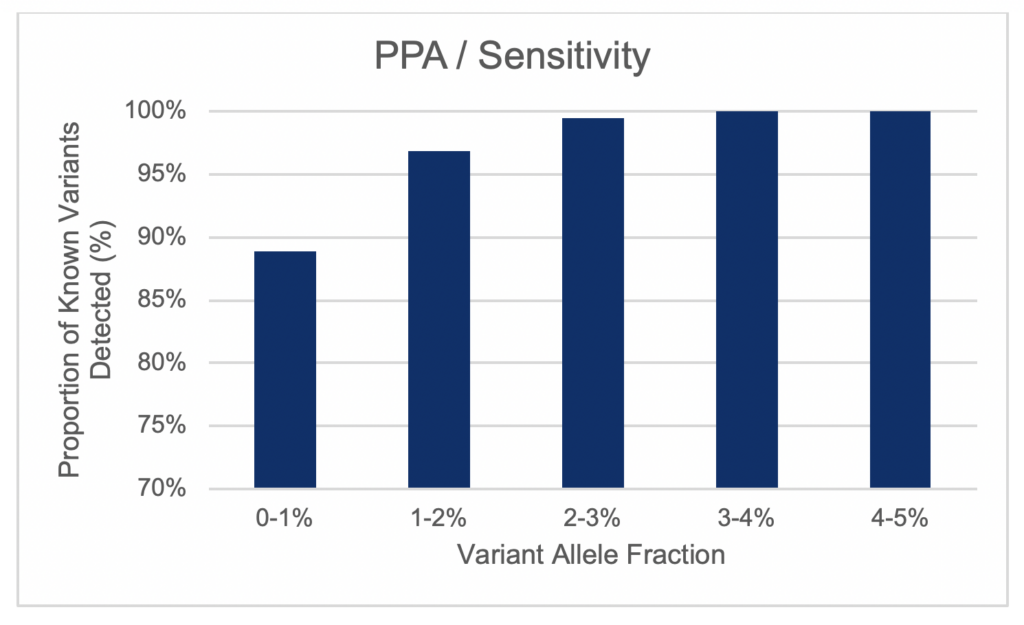
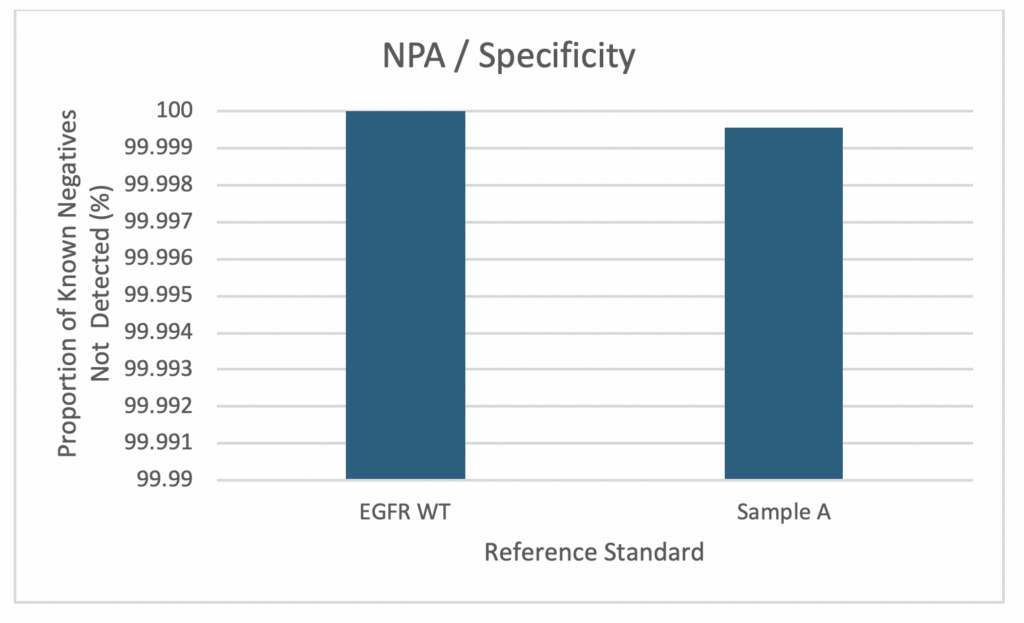
Scientist at Frontage have combined superior a library preparation and hybridization workflow with a customized, state-of-the-art bioinformatics pipeline to deliver highly accurate and reliable data and variant calls. Our assay is able to detect somatic variants with a variant allele frequency (VAF) below 1% and has a very low false positive rate (< 5 false positives per 1,000,000 bp).
High Sensitivity of cfDNA NGS LDT
Low False Positive Rate cfDNA NGS LDT
We offer superior bioinformatics analysis and personalized genetic reports

Validated for Clinical Use under CLIA/CAP
Our NGS Oncology Panel and cfDNA NGS workflow is validated for clinical use under CLIA/CAP.
Providing insight into cancer through DNA sequence analysis
Resources To Consider


Cell-Free DNA NGS Testing

Liquid Biopsy: Challenges and benefits of cell-free DNA sequencing

Validating Digital Droplet PCR (ddPCR) Assay with Zero-Inflated…




